PDAI CALCULATION:
- Home
- / PDAI CALCULATION:
◼️ *TOACS Station – Pemphigus Disease Area Index (PDAI) Calculation and Consent*
VIVA QUESTIONS:
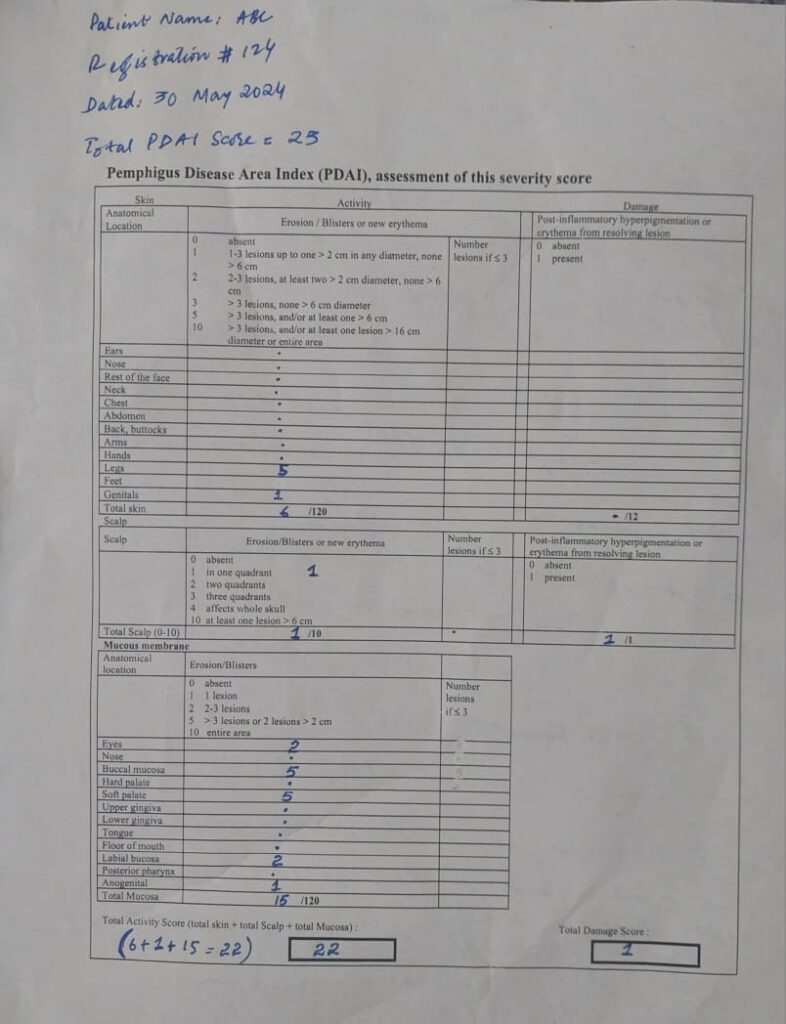
🔘🔘 *PDAI – Qs & As* 🔘🔘🔮 *Q*: What is the PDAI?
☑️ *A*: PDAI is a validated scoring system used to assess the severity and extent of pemphigus vulgaris and pemphigus foliaceus. It measures disease activity based on the presence and extent of lesions in various body regions, including the skin, scalp, and mucous membranes.
🔮 *Q*: How is the PDAI structured, and what does it evaluate?
☑️ *A*: The PDAI is divided into three main sections:
1. *Skin Activity Score*: Evaluates the number and size of lesions on the skin.
2. *Scalp Activity Score*: Assesses the involvement of lesions on the scalp.
3. *Mucosal Activity Score*: Measures the extent and severity of lesions in mucous membranes (oral, ocular, nasopharyngeal, genital).
◼️ Each section assigns scores based on the extent and severity of lesions, with higher scores indicating more severe disease activity.
🔮 *Q*: What is the scoring range for the PDAI, and how is it interpreted?
☑️ *A*: The total PDAI score ranges from 0 to 263 points, with higher scores indicating more severe disease activity. The breakdown is as follows:
– *Skin Activity Score*: 0 to 120
– *Scalp Activity Score*: 0 to 10
– *Mucosal Activity Score*: 0 to 120
– *Disease Damage Score*: 0 to 13
◼️ A higher total PDAI score reflects a greater extent and severity of pemphigus lesions, aiding in monitoring disease progression and response to treatment.
🔮 *Q*: How is the PDAI used in clinical practice?
☑️ *A*: In clinical practice, the PDAI is used to:
• Assess baseline disease severity.
– Monitor changes in disease activity over time.
– Evaluate the effectiveness of therapeutic interventions.
– Guide treatment decisions based on disease severity and progression.
◼️ Regular PDAI assessments help clinicians tailor treatment plans to individual patient needs and track therapeutic outcomes.
🔮 *Q*: What are the benefits of using the PDAI in managing pemphigus?
☑️ *A*: The benefits of using the PDAI include:
– *Standardization*: Provides a standardized method for assessing disease severity.
– *Objectivity*: Offers an objective measure to evaluate disease activity.
– *Treatment Monitoring*: Facilitates monitoring of treatment efficacy and adjustment of therapeutic strategies.
– *Research Utility*: Useful in clinical trials to compare the efficacy of different treatments.
🔮 *Q*: Are there any limitations to the PDAI?
☑️ *A*: While the PDAI is a valuable tool, it has some limitations:
– *Complexity*: Scoring can be time-consuming and requires training for accurate assessment.
– *Subjectivity*: Some degree of subjectivity may still be present in scoring certain lesions.
– *Limited Scope*: Focuses primarily on disease activity and does not fully capture patient-reported outcomes or quality of life measures.
🔮 *Q*: How does the PDAI compare to other pemphigus scoring systems, such as the Autoimmune Bullous Skin Disorder Intensity Score (ABSIS)?
☑️ *A*: The PDAI is specifically designed for pemphigus and provides a detailed assessment of disease. The ABSIS is another scoring system that evaluates both disease activity and the impact on daily activities.
◼️ While ABSIS includes quality of life measures, the PDAI offers a more granular assessment of lesion severity and extent, making it particularly useful for monitoring clinical changes and treatment response.
◼️Both systems have their unique strengths and can be complementary in comprehensive disease assessment.
IMPORTANT POINTS:
📌As pointed out,this score is a bit complicated and detailed so practicing it on a couple of patients will help to be fluent in this.
📌Mucosal examination needs to be detailed and consent should be taken to examine all the hidden areas with proper privacy and dignity of the patient maintained.
📌Estimate of severity will help in guiding the appropriate treatment as well as response to treatment on follow up visits so proper record needs to be maintained for each patient with complete biodata and date with time mentioned on the forms.
SAMPLE: